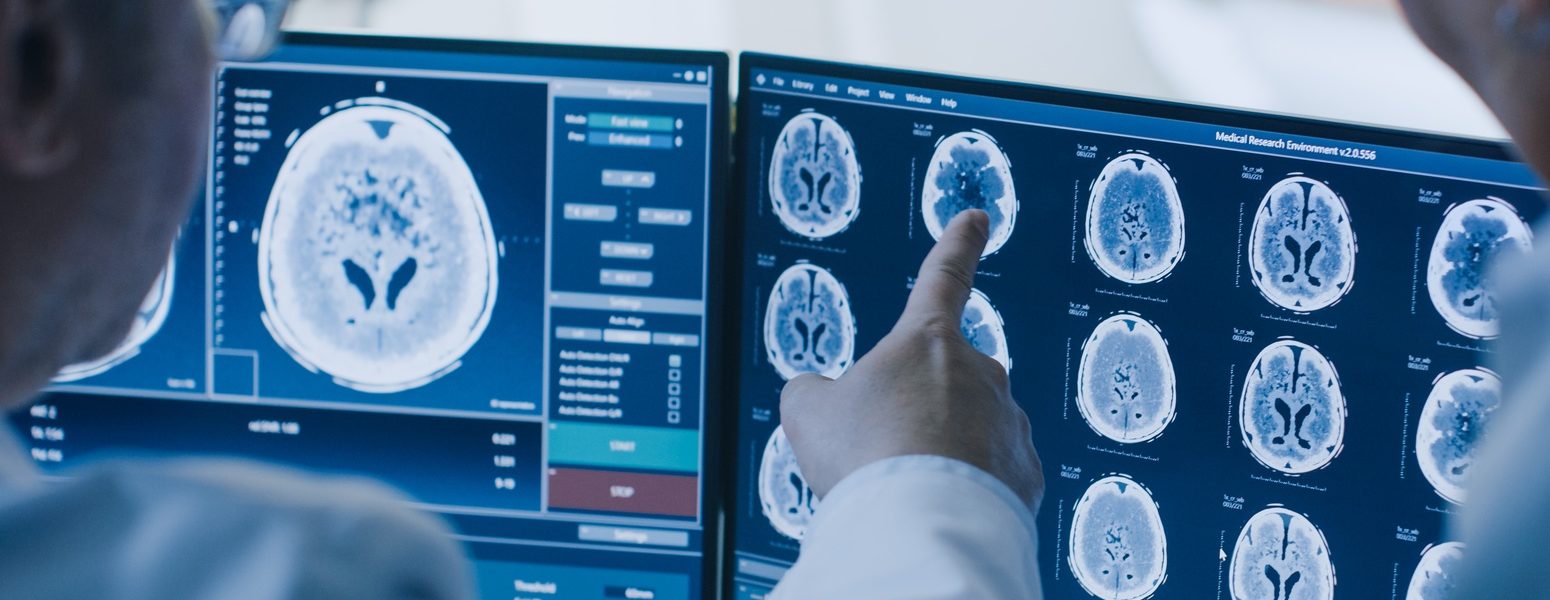
Neuroscience clinical trials
Significant unmet needs persist in the treatment and management of neurological diseases. Yet, recruiting and enrolling qualified patients to participate in these clinical trials remains a difficult challenge.
Why AES for neuroscience clinical trials?
Through our Patient Recruitment solution, we bring extensive expertise recruiting patients for clinical trials studying a diverse range of neurological diseases, including migraine, Alzheimer’s disease, Parkinson’s disease and psychiatric conditions such as depression.
In addition, if you choose our Integrated Network & Patient Recruitment solution, which expands on our recruitment solution and recruits volunteers and patients directly into AES’s Clinical Research Network, you gain access to neurologists and psychiatrists in 11 countries across the globe with extensive neuroscience experience, including five specialized U.S.-based neurology/psychiatry sites.
AES Experience in Neuroscience
Alzheimer’s disease
Despite the urgent need for treatments for Alzheimer’s disease and other forms of dementia, finding patients for these clinical trials can be frustrating for a variety of reasons. Because the science suggests that early treatment may offer the best approach to helping to slow or even reverse cognitive decline, cognitive studies often need patients with mild cognitive impairment. Yet prodromal or mildly impaired patients often do not seek a diagnosis—let alone participation in a clinical study.
In order to better understand those factors influencing brain aging and cognitive decline – and build a pool of otherwise healthy individuals for cognition research trials —we have created the Synexus HealthyMinds Registry. This five-year study is recruiting 30,000 subjects in the U.S. to examine the lifestyle factors impacting cognitive function over time. In addition, our proprietary database includes over a million trial-ready patients with cognitive decline due to Alzheimer’s disease or other forms of dementia.
AES has conducted over 80 Phase II and Phase III Alzheimer’s/dementia trials during the past five years and has access to neurologists at over 30 sites globally.
Migraine
The migraine clinical trial landscape has shifted substantially in recent years. New medications and an accelerating volume of trials are generating increased demand for patients in an already crowded field. The traditional model of relying on sites to find patients – which is inefficient and ill-equipped to meet the needs of most trials – is particularly ineffective for migraine trials, where the pool of treatment-naive or difficult-to-treat patients is limited.
With a proprietary database of more than 10 million patients self-reporting a migraine condition, we are one step ahead of other clinical trial patient recruitment companies that are relying on third-party databases.
In the past five years, AES has delivered on more than 60 migraine studies for both chronic and episodic migraines, and randomized more than 4,000 patients. As a result of our extensive migraine capabilities and expertise, we’ve more than doubled enrollment rates on trials we’ve supported.
AES neuroscience ambassador teams
Discuss medical and science issues and review new and ongoing trials across AES sites
Neuroscientists who are part of the network and bring significant expertise in their field
Best practices and insights for running neuroscience trials
Parkinson’s disease
There is a great unmet need for Parkinson’s disease treatments, and once diagnosed, Parkinson’s disease patients are often highly motivated to join trials. However, narrow inclusion/exclusion criteria have led to great challenges recruiting and enrolling patients. For example, studies may seek patients early in the disease who are not receiving a disease-modifying treatment. However, PD patients with few or no symptoms may attribute their tremors, for example, to normal aging, and thus would not yet be diagnosed, let alone consider enrolling in a clinical trial.
Our Parkinson’s disease experience includes studies conducted in the past five years across various phases in a broad spectrum of populations ranging from early to advanced Parkinson’s disease and including gene mutation populations.
Psychiatric indications
Over the past five years, we have conducted studies in indications ranging from schizophrenia to anxiety to depression, expertly overseeing global programs of all sizes.
We offer our customers access to a strong network of qualified global resources, including our global investigator database of psychiatrists, neurologists, neurological surgeons and gerontologists, opening access to local referral networks across geographies and ages.
We are very familiar with the common pitfalls these sensitive studies may encounter. Cultural differences, as one example, can pose significant challenges to global programs. A patient in the United States may rate their symptoms differently than a patient with a similar burden of disease in Poland. Reducing the number of raters conducting assessments is one strategy to reduce data variability. AES Integrated Network & Patient Recruitment solution helps minimize the research site footprint by recruiting patients directly into our Clinical Research Network that are purpose-built to support large volumes of patients and staffed by experienced teams dedicated exclusively to clinical research.
Our centrally managed research site solution also introduces more consistency through standardized processes to deliver uniform site conduct across sites, reducing risk. And several of our sites also offer inpatient psychiatric facilities if needed.

