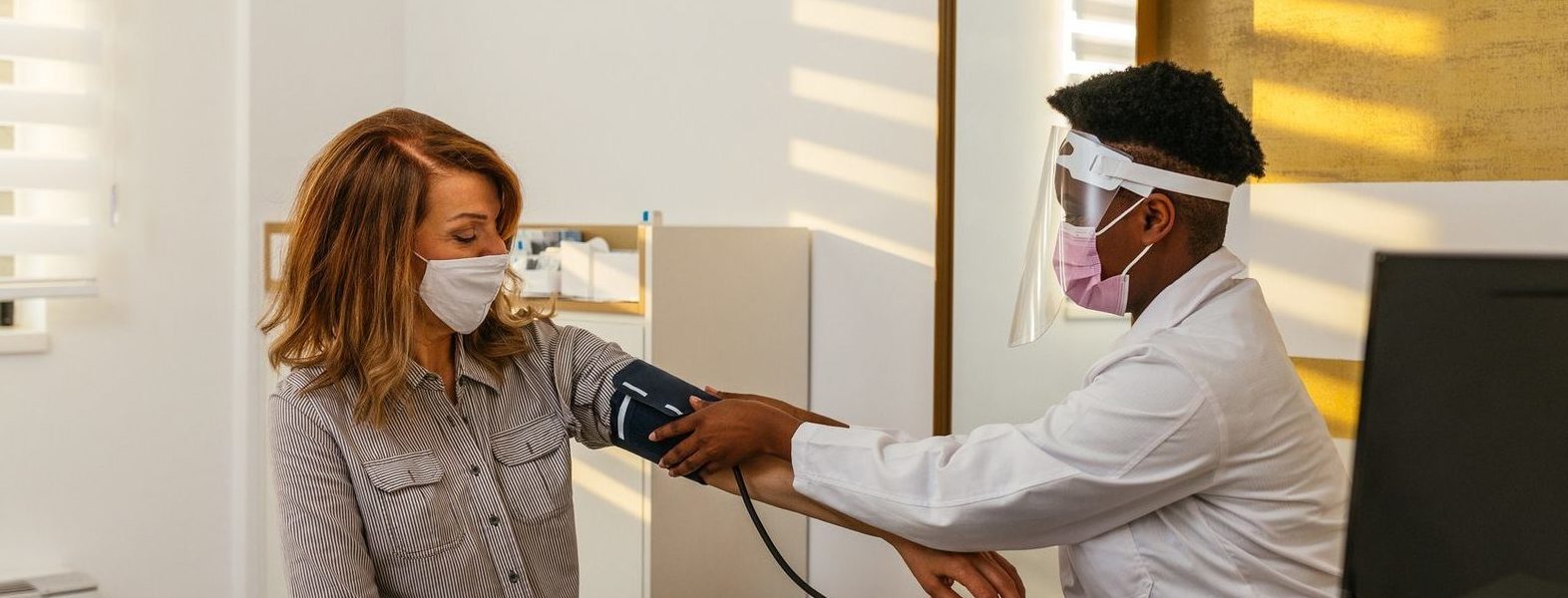
Cardiovascular clinical trials
Cardiovascular clinical trials are usually complex, often competitive, and almost always challenging. Finding, engaging, recruiting and retaining vulnerable cardiovascular patient populations, especially since the advent of COVID-19, is a formidable task.
Why AES for cardiovascular clinical trials?
AES brings the precise mix of expertise, insights, specialists and infrastructure necessary to overcome these challenges. We know that the details make the difference for a patient population that may find the demands of a clinical trial to be particularly burdensome. Identifying qualified patients near but unknown to research sites, for example, helps ease travel burdens. Having cardiologists on site who deeply understand the patient journey improves the patient experience. And having AES sites situated in the community—not at hospitals—brings more comfort and accessibility to a patient population at high risk for potential COVID-19 complications.
Other key AES differentiators include:
AES Experience
Featured indication: Heart failure clinical trials
When we employ our solutions for heart failure studies, we also bring to bear our resources, experience and expertise developed specifically for this indication. We’ve randomized nearly 100 patients in six studies around the world at more than 80 research sites over the past five years.
This experience doesn’t just give us unmatched expertise – it gives us the data we need to create recruitment plans that we know will generate results for studies, and the confidence to back up our results with performance-based pricing. We also glean the insights to more accurately identify the patients most likely to enroll , including those outside of the small, depleted databases of confirmed heart failure patients at specialist sites.
The diagnosis of heart failure is often challenging. Symptoms may be nonspecific, and signs may be absent or difficult to interpret, particularly in an outpatient setting. Our proprietary database not only includes 1.1 million heart failure trial candidates, but also includes tens of millions of candidates with risk factors that are highly predictive of a randomized heart failure patient, including 30 million candidates over age 60, over 20 million with obesity, over 8 million with type 2 diabetes and over 6 million with hypertension.
Not only do we engage with the vast pool of undiagnosed individuals whose comorbid conditions place them at a high risk of heart failure, but we also leverage databases from our global network of cardiologist partners. Our ability to model the more probable patient results in improved quality of patient referrals, reducing site burden.
In addition, selecting our Integrated Network & Patient Recruitment solution means that we recruit volunteers and patients directly into our Clinical Research Network. We can also employ 55 research sites across the world with clinical research teams who have previously run heart failure clinical trials. Our network includes ample access to more than 70 cardiologists acting as principal investigators who are seasoned in advising on clinical trial protocols and in conducting clinical trials in this specialty.
As a result of our heart failure capabilities and expertise, we’ve at least doubled enrollment rates on the trials we’ve supported.
Targeted outreach strategies in specific communities
We have proven methodologies to engage diverse populations often underrepresented in access to medical treatment and clinical research. For these groups, participating in a clinical trial is a burden that can be particularly acute. That’s why our messaging and creative is specifically tailored to take into account the perceptions and needs of diverse populations, including their attitudes toward clinical research and their motivations and barriers to become involved.
In addition to traditional outreach via social media, direct mail, etc., we access volunteers through familiar, local and well-trusted connections within their community (e.g., outreach to community- and faith-based organizations), as well as through our global network of providers and healthcare systems. We then offer flexible scheduling, virtual first office visits and minority investigators/staff at the site to improve the volunteer experience.

